EON
EON is an electrostatics comparison program. It compares electrostatic potential maps of pre-aligned molecules and determines the Tanimoto measures for the comparison.
As the electrostatic potential of molecules is not strongly linked to the molecular graph, EON can screen molecular databases for electrostatic similarity to a lead compound to identify lead-hops.
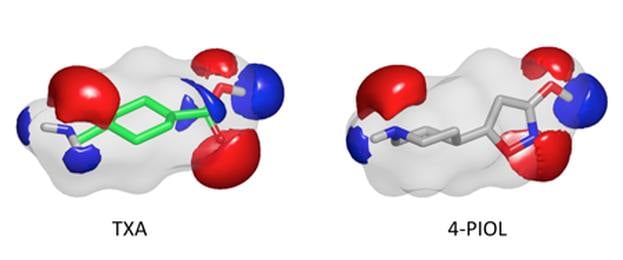
In particular, the electrostatic comparison program EON has shown promise in identifying leads and tool compounds… -- Boström et al. (J. Med. Chem. 2013)
Features
- Identifies molecules with high electrostatic similarity
- Reports rigorous Tanimoto measure between electrostatic grids
- Offers various charge models; user-defined models may also be used
- Integrates well with ROCS®
- Distributed processing via MPI for all supported platforms
- Available as both an application and a toolkit, as well as on the cloud

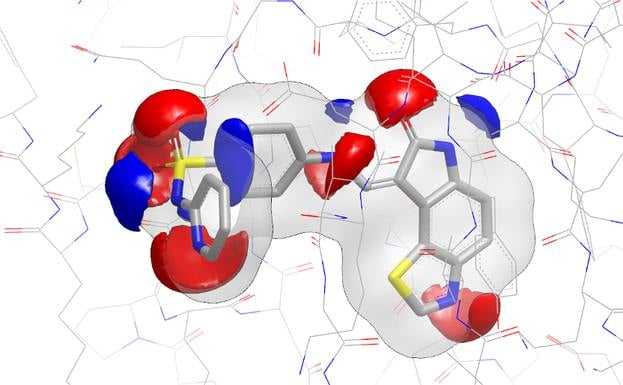
Since the input molecules must already be aligned, EON is suitable for analyzing the output structures from programs such as ROCS, thus yielding compounds with similar shape and electrostatics.
EON contributes significantly to lead generation and library design. In a published study, Abbott scientists reported that EON's post-processing of ROCS hits helped them lead-hop a MCH-1 series that had demonstrated in vivo potency issues [1]. In a chemical genomics project directed toward antagonists for NAADP, EON was used in complement to ROCS to identify a low nanomolar binder to the NAADP receptor [2].
For more detailed information on EON, check out the link below:
DocumentationReferences
- The Use of Three-Dimensional Shape and Electrostatic Similarity Searching in the Identification of a Melanin-Concentrating Hormone Receptor 1 Antagonist S. Muchmore, A. J. Souers, I. Akritopoulou-Zanze, Chem. Biol. Drug Des., 2006, 67, 174.
- Identification of a chemical probe for NAADP by virtual screening E. Naylor, A. Arredouani, S.R. Vasudevan, A.M. Lewis, R. Parkesh, A. Mizote, D. Rosen, J.M. Thomas, M. Izumi, A. Ganesan, A. Galione and G.C. Churchill, Nature Chemical Biology, 2009, 5, 280-292.
- Potent Fibrinolysis Inhibitor Discovered by Shape and Electrostatic Complementarity to the Drug Tranexamic Acid Jonas Boström, J. Andrew Grant, Ola Fjellström, Anders Thelin, David Gustaffson, J. Med Chem., 2013, 56, 3273-3280.
miniCUP Cambridge 2025
Cadence Announces New Life Sciences Leadership
Expanding Orion’s Capabilities with AI
miniWebinar: Faster, Larger, Smarter: Filling the Funnel for Ultra-Large Scale Virtual Screening
Resources
Glimpse the Future through News, Events, Webinars and more
Event
miniCUP Basel 2025
Event
miniCUP Cambridge 2025


